The term “Carbon Sequestration” refers to processes, either natural or artificial, in which carbon dioxide is removed from the atmosphere, captured, secured, and stored in solid or liquid form. Carbon dioxide is a heat-trapping greenhouse gas that comes from both natural and human activities. Natural sources of carbon dioxide include animal exhalation, decomposing vegetation, outgassing from the ocean, and volcanoes. Human-generated carbon dioxide comes primarily from energy production, including burning coal, oil, or natural gas, and cement production. The build-up of carbon dioxide and other greenhouse gases in the atmosphere can trap heat and contribute to climate change. The capture and long-term storage of carbon dioxide in solid and dissolved forms is now recognized as a key part of a comprehensive climate change mitigation strategy to limit the amount of human-made carbon dioxide contributed to Earth’s atmosphere.
.png)
Schematic of carbon dioxide from a Direct Air Capture facility being stored in various geologic formations. Figure modified from Pacific Northwest National Laboratory and Climeworks.
The three types of carbon sequestration
- Natural or biological carbon sequestration occurs where carbon dioxide is captured, secured, and stored in land-based vegetation, soils, and oceans. On land, carbon dioxide from the atmosphere is collected by trees and plants through photosynthesis and stored as carbon in soils and biomass (tree trunks, branches, foliage, and roots). Large volumes of carbon dioxide are also absorbed, released, and stored by the oceans. Increasing the amount of carbon dioxide stored in the ocean may be accomplished by increasing the productivity of ocean biological systems by iron fertilization or by pumping carbon dioxide into the deep sea.
- Technological carbon sequestration uses innovative mechanical processes to capture, secure, and store carbon dioxide either at the point of emission (point source capture), or directly from the atmosphere (direct air capture (DAC)). The captured carbon dioxide can then be injected into the ground to be mineralized or may be reused for secondary industrial purposes such as food and beverage, greenhouse horticulture, and materials manufacture (fuels and plastics).
- Geologic carbon sequestration is the process in which carbon dioxide is captured and then stored in underground geologic rock formations. Carbon dioxide may be stored in a variety of places, including oil and gas reservoirs, saline aquifers, unmineable coal seams, high-organic-content shale formations, and basalt. Typically, carbon dioxide is captured from an industrial source, such as a power plant or natural gas processing facility, converted into a liquid, and injected deep (thousands of feet) into porous rocks for long-term storage. Once underground, the carbon can be safely and permanently stored.
Carbon dioxide emissions in Oregon
According to the Oregon Department of Environmental Quality, Oregon’s emissions of carbon dioxide are approximately 60 to 70 million tons annually. In 2021, Oregon released 61.4 million tons of carbon dioxide. Oregon emissions have been relatively consistent in the period of measurement between 1990 and 2021. The top sources of carbon dioxide are transportation (cars, boats, planes, trains), heating for homes and workplaces, industrial applications, and agriculture.
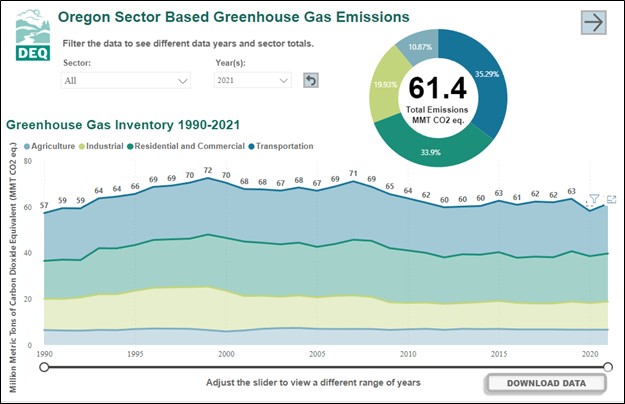
Graph showing Oregon's greenhouse gas inventory by type: transportation, heating for homes and workplaces, industrial applications, and agriculture between 1990 and 2021. Total emissions during this time period were relatively constant averaging around 65 million metric tons per year.
Credit:Oregon DEQ, Action on Climate Change.
Point Sources
Point sources are fixed structures where carbon dioxide is emitted. Point source emissions account for about half of all Oregon carbon dioxide emissions. The remaining emissions come from vehicle tailpipes and are harder to estimate, let alone to physically capture.
Point sources are grouped along the Columbia River/Interstate-84 corridor in northern Oregon and southern Washington State. Others are spread out across the western half of the state of Oregon.
Point sources include:
• Power plants (coal and natural gas)
• Refineries, chemical productions, oil and gas pipeline transmission
• Paper and pulp mills
• Metal, mineral, and cement production and processing
• Other (waste incinerators, agricultural processing, military, universities, technology manufacturing)
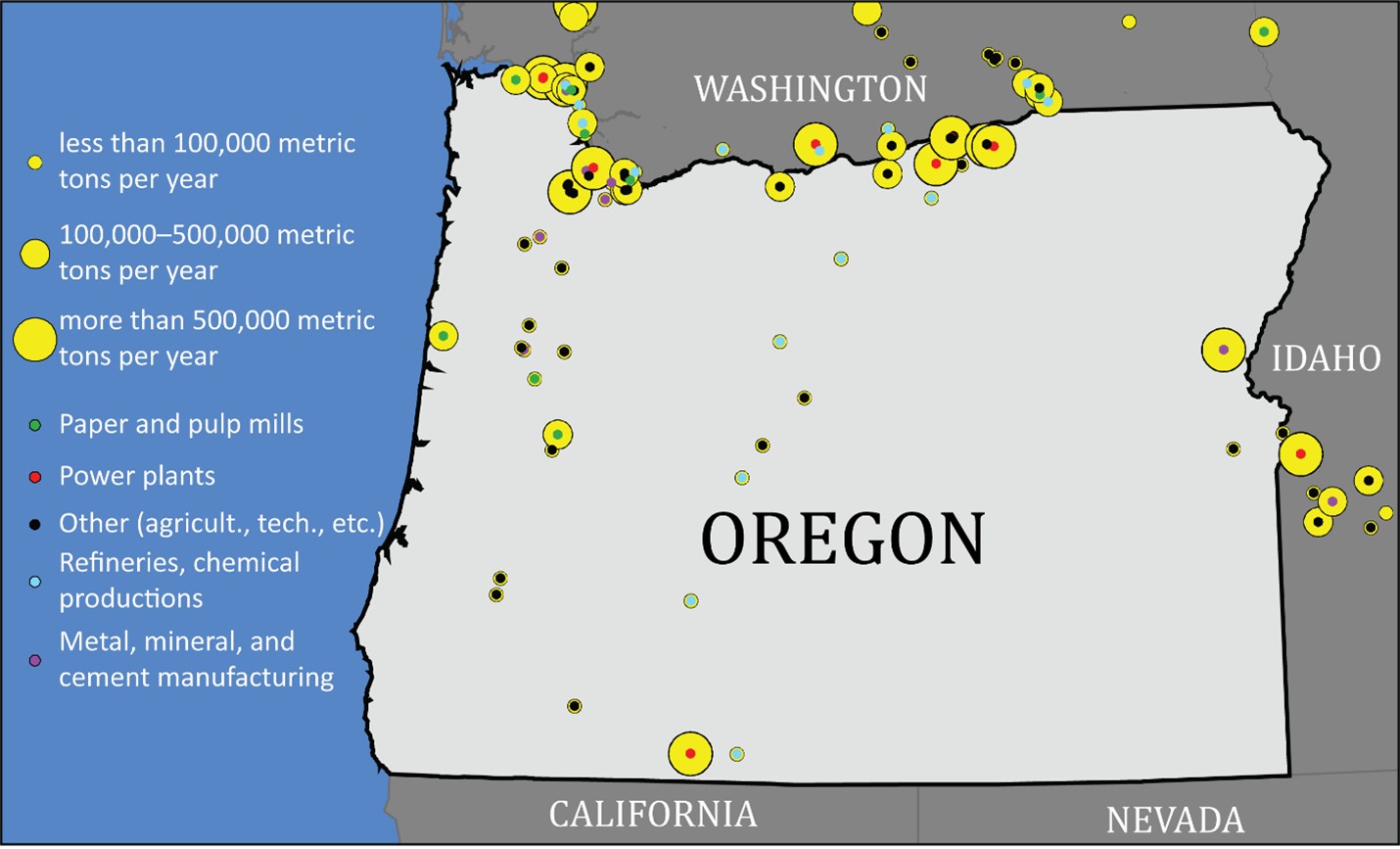 Map of carbon dioxide point sources in Oregon as of 2021. The graphic includes nearby sources in Washington and Idaho. Larger yellow circles indicate bigger sources of carbon dioxide. Credit: Data from the
Environmental Protection Agency Data Sets. Click the image to expand the map.
Map of carbon dioxide point sources in Oregon as of 2021. The graphic includes nearby sources in Washington and Idaho. Larger yellow circles indicate bigger sources of carbon dioxide. Credit: Data from the
Environmental Protection Agency Data Sets. Click the image to expand the map.
Geologic carbon sequestration possibilities in the Pacific Northwest
Western Oregon basins include areas of historic oil and gas exploration in the southern Willamette Valley, Chehalem Valley southwest of Portland, west of St. Helens, and Astoria Basin, including the Mist Gas Field (U.S. Geological Survey Geologic Carbon Dioxide Storage Resources Assessment Team, 2013).
Basin areas of northwest Oregon are underlain by a variety of bedded marine rocks including the Eocene Spencer, Yamhill, and Tyee Formations and Oligocene-Miocene Astoria and Scappoose Formations (Franczyk and others, 2020). These rocks are in turn overlain or invaded by younger lava flows of the Wanapum and Grande Ronde Basalt of the early to middle Miocene Columbia River Basalt Group (CRBG).
Western Oregon and Washington Basins collectively have estimated technically accessible storage resources of 14,000 megatons of carbon dioxide (U.S. Geological Survey Geologic Carbon Dioxide Storage Resources Assessment Team, 2013). Technically accessible storage resources are those that can be accessed using today's technology and pressurization and injection techniques.
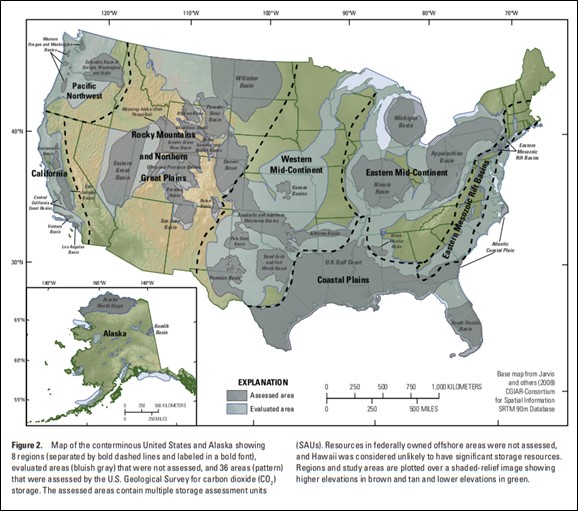
Credit: U.S. Geological Survey Geologic Carbon Dioxide Storage Resources Assessment Team (2013)
Mafic rocks, particularly extensive stacked basaltic lava flows, are significant potential reservoirs for carbon dioxide storage and mineralization (Blondes and others, 2019). Thick and deep lava flow sequences of the 17 to 6 million-year-old Columbia River Basalt Group (CRBG), a continental flood-basalt province, serve as potential reservoirs for carbon dioxide storage and mineralization in the Columbia Basin of eastern Oregon and Washington (Blondes and others, 2019; Franczyk and others, 2020).
Target reservoirs in the CRBG lie within the Grande Ronde Basalt in the Columbia Basin. These rocks have been the focus of many studies on carbon dioxide storage and mineralization. The Grande Ronde Basalt is composed of ~100 individual, laterally extensive, basalt and basaltic andesite lava flows, encompassing an area of nearly ~65,599 square miles, a thickness up to 14,760 feet, and a volume of approximately 36,083 cubic miles (Reidel and Tolan, 2013). Grande Ronde lava flows consist of highly fractured, weathered, brecciated, and/or vesicular flow tops and bases with dense, crystalline colonnade and/or entablature interiors. Lava flow tops and bottoms have an estimated porosity of 14 to 39%, while most flow interiors are estimated to have a porosity around 1 to 2% (Sublette, 1986; Whiteman and others, 1994; Reidel and others, 2002; Cao and others, 2023). Estimates of carbon dioxide storage potential in the CRBG range from 10 metric gigatons (Gt) to 100 Gt (McGrail and others, 2006; Gislason and others, 2010).
Geologic carbon sequestration is not the only type of storage project proposed in the CRBG. The CRBG has also been investigated for natural gas storage, as a possible nuclear waste storage site in the vicinity of Hanford, WA, for reservoir thermal energy storage (RTES) in the Portland Basin, and for low temperature geothermal resources across the Columbia Basin that include potential for energy production. Several cities in Oregon, including Beaverton, Cornelius, Salem, and Pendleton each have existing aquifer storage and recovery (ASR) projects that utilize the basalt interflow zones for seasonal storage of potable water.
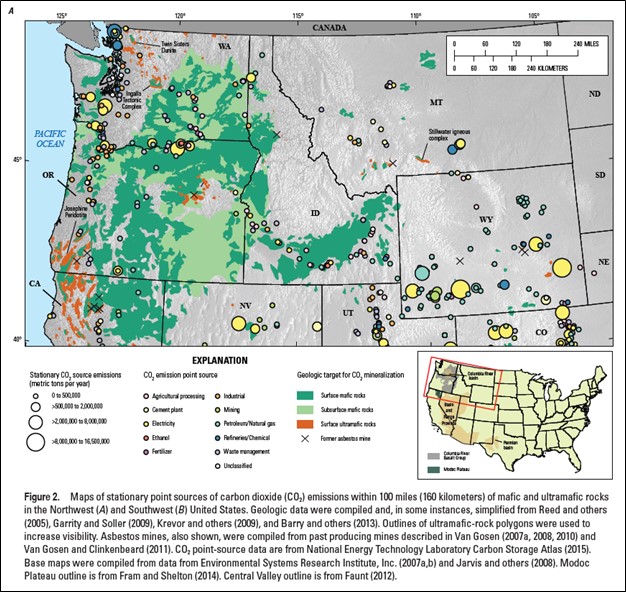
Credit: Blondes and others (2019)
Extent of CRBG in Oregon, Washington, and Idaho and a schematic section of typical CRBG lava flows. Targets for carbon dioxide storage would be the porous tops of lava flows.
References
Blondes, M.S., Merrill, M.D., Anderson, S.T., and DeVera, C.A., 2019, Carbon dioxide mineralization feasibility in the United States: U.S. Geological Survey Scientific Investigations Report 2018–5079, 29 p.,
https://doi.org/10.3133/sir20185079.
Cao, R.; Miller, Q. R. S.; Davidson, C. A.; Gallin, W.; Reidel, S. P.; Jiao, Z.; McLaughlin, F.; Schaef, H. T., 2023 (in review), Gigaton commercial-scale carbon storage and mineralization potential in stacked Columbia River Basalt reservoirs: Energy and Fuels. 12 p.
Franczyk, J. J., Madin, I. P., Duda, C. J. M., and McClaughry, J. D., 2020, Oregon geologic data compilation, release 7 [OGDC-7] (statewide): Oregon Department of Geology and Mineral Industries Digital Data Series OGDC-7, Esri geodatabase.
https://pubs.oregon.gov/dogami/dds/p-OGDC-7.htm
Gislason, S.R., Wolff-Boenisch, D., Stefansson, A., Oelkers, E.H., Gunnlaugsson, E., Sigurdardottir, H., Sigfusson, B., Broecker, W.S., Matter, J.M., Stute, M., Axelsson, G., and Fridriksson, T., 2010, Mineral sequestration of carbon dioxide in basalt—A pre-injection overview of the CarbFix project: International Journal of Greenhouse Gas Control, v. 4, no. 3, p. 537–545.
McGrail, B.P., Schaef, H.T., Ho, A.M., Chien, Y.-J., Dooley, J.J., and Davidson, C.L., 2006, Potential for carbon dioxide sequestration in flood basalts: Journal of Geophysical Research, v. 111, no. B12, 13 p.
Reidel, S. P.; Tolan, T. L., 2013, The Grande Ronde Basalt, Columbia River Basalt Group. In Reidel, S. P.; Camp, V. E.; Ross, M. E.; Wolff, J. A.; Martin, B. S.; Tolan, T. L.; Wells, R. E., editors, The Columbia River flood basalt province: Geological Society of America Special Paper v. 497, p. 117-154.
Reidel, S. P.; Johnson, V. G.; Spane, F. A., 2002, Natural gas storage in basalt aquifers of the Columbia Basin, Pacific Northwest USA--A guide to site characterization: Pacific Northwest National Laboratory PNNL-13962, 1 v.
U.S. Geological Survey Geologic Carbon Dioxide Storage Resources Assessment Team, 2013, National assessment of geologic carbon dioxide storage resources—Results (ver. 1.1, September 2013): U.S. Geological Survey Circular 1386, 41 p., http://pubs.usgs.gov/circ/1386/. (Supersedes ver. 1.0 released June 26, 2013.)
Whiteman, K. J.; Vaccaro, J. J.; Gonthier, J. B.; Bauer, H. H., 1994, The hydrogeologic framework and geochemistry of the Columbia Plateau aquifer system, Washington, Oregon, and Idaho: U.S. Geological Survey Professional Paper 1413-B, 73 p.