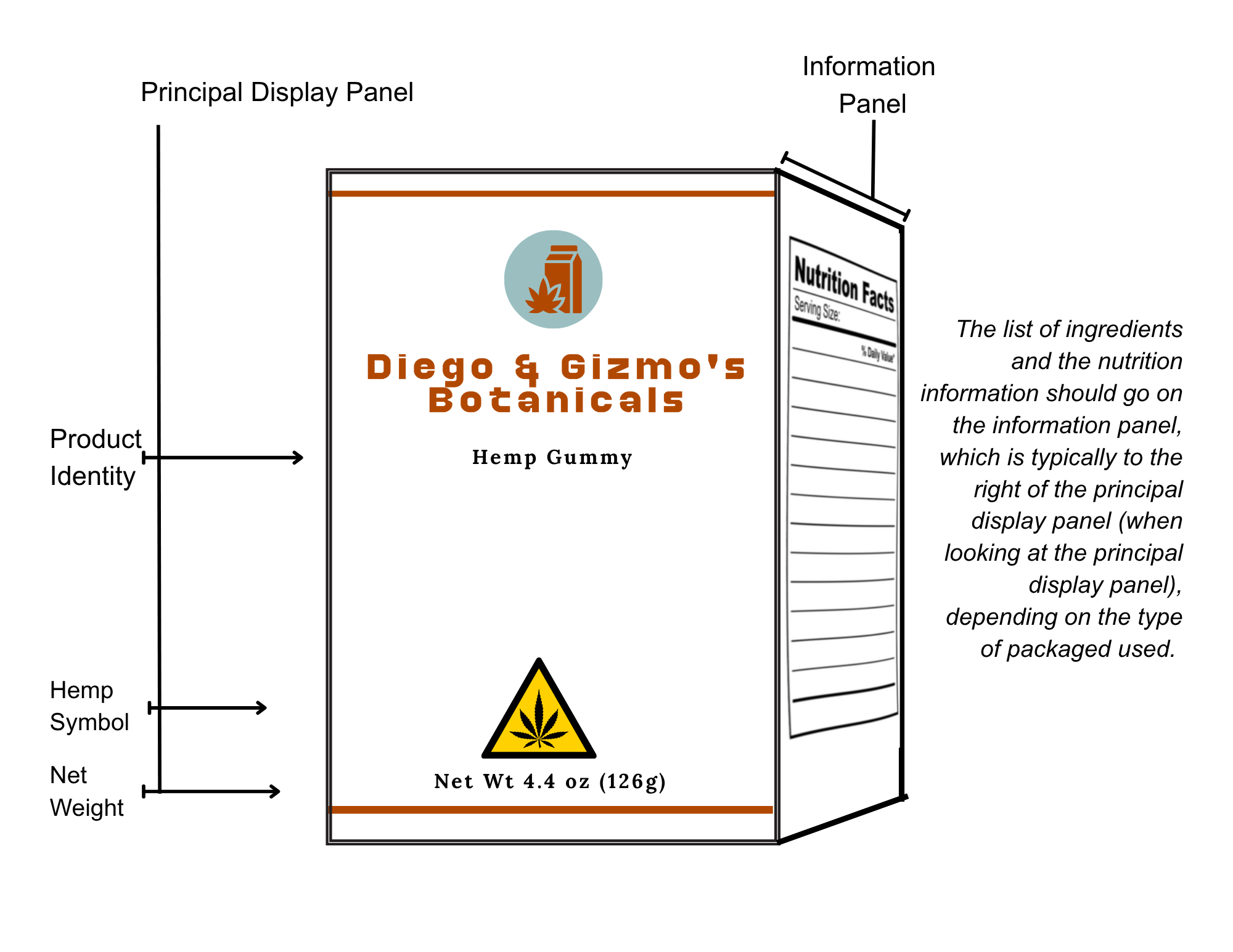
For hemp edibles, it is required that the following information be placed on the label:
1. List of all ingredients in descending order of predominance by weight or volume used to process the hemp edible. The list of ingredients must include any substance used in processing, preparing, manufacturing, packaging, or holding the hemp edible product is present in the final product, including any cooking or release spray. The list of ingredients must correctly identify the type of hemp ingredient used to make the product. For example: “hemp extract” or “hemp concentrate.”
This includes all ingredients and sub ingredients. For example, in a chocolate chip cookie recipe, the ingredient list may be as follows: Ingredients: Enriched Flour, Brown Sugar, Chocolate Chips, Cottonseed Oil, Baking Soda, Salt.
However, both enriched flour and chocolate chips are composed of other sub-ingredients. For this example, the chocolate chips are made of cane sugar, chocolate liquor, cocoa butter, milkfat, and soy lecithin and the enriched flour is made of wheat flour, malted barley flour, niacin, iron, thiamin mononitrate, riboflavin, and folic acid. Because these two ingredients have ingredients of their own, you must list all of the ingredients and sub-ingredients in one of two ways:
First, you can list the names of the ingredients and then list any sub ingredients in parenthesis: Ingredients: Enriched Flour (Wheat Flour, Malted Barley Flour, Niacin, Iron, Thiamin Mononitrate, Riboflavin, Folic Acid), Brown Sugar, Chocolate Chips (Cane Sugar, Chocolate Liquor, Cocoa Butter, Milkfat, Soy Lecithin), Cottonseed Oil, Baking Soda, Salt.
Second, you could list out each ingredient in descending order of predominance by weight or volume: Ingredients: Wheat Flour, Malted Barley Flour, Niacin, Iron, Thiamin Mononitrate, Riboflavin, Folic Acid, Brown Sugar, Cane Sugar, Chocolate Liquor, Cocoa Butter, Milkfat, Soy Lecithin, Baking Soda, Salt.
The amount of calories, sodium, protein, added sugars, cholesterol, total carbohydrates, and total fat per serving. A hemp edible shall use one of the nutrition information formats provided by the Commission to display the amount of calories, sodium, protein, added sugars, cholesterol, total carbohydrates, and total fat per serving, the serving size and number of servings per container, and the list of ingredients and potential allergens. Even if the amount per serving is zero, it must still be listed on the label.
The nutrition templates can be found here.
The rules do not require a specific type of analysis to determine the nutrient amounts displayed in the Nutrition Facts Panel but you may use one of the following methods to determine those values:
A. Database analysis. Using a list of ingredients and specific processing information for your product, you can use a food ingredient database to determine the specific nutrient amounts for your product. This method may be a better predictor of nutrient values across multiple batches versus a single laboratory test from one batch. However, how the analysis is performed affects the validity of the results. You must be extremely detail-oriented and have a general knowledge of food and nutrient values, be able to understand and account for processing changes, and be able to keep detailed records.
B. Lab analysis. A lab can determine the nutrient values for the sample submitted to the lab. The results will only be specific to the sample tested so you may want to consider testing your product throughout the year to get the most accurate results. Lab analysis may be more beneficial if you are using unique ingredients that do not have nutrient information available or when the specific process you are using to make the edible is going to change the nutrient composition of the product in an unpredictable way.
C.
A combination of database and lab analysis. You can verify a claim or cross check results using both methods.
2. If the edible is perishable, a statement that the edible must be refrigerated or kept frozen. If the edible is not perishable, no statement is needed.
3. List of potential major food allergens.
- The label must list major food allergens if the edible contains:
- Milk, egg, fish, crustacean shellfish, tree nuts, wheat, peanuts, soybeans, or sesame as an ingredient; or
- Any ingredient that contains protein derived from: milk, egg, fish, crustacean shellfish, tree nuts, wheat, peanuts, or soybeans.
When labeling allergens, always use the specific food name for nuts, fish or crustacean shellfish and not the category of allergen. For example, use the word “almonds” instead of “tree nuts” in the Contains statement. Major food allergens must be labeled in one of two ways.
The first option is to include the name of the food source in parenthesis following the common or usual name of the major food allergen in the list of ingredients whenever the name of the food source of the major allergen does not appear elsewhere in the ingredient statement. For example: Ingredients: Enriched flour (wheat flour, malted barley, niacin, reduced iron, thiamin mononitrate, riboflavin, folic acid), sugar, partially hydrogenated
soybean oil, and/or cottonseed oil, high fructose corn syrup, whey (milk),
pecans, eggs, vanilla, natural and artificial flavoring) salt, leavening (sodium acid pyrophosphate, monocalcium phosphate), lecithin (soy), mono-and diglycerides (emulsifier)
In the example above, the major food allergens are in bold to highlight their location. However, the allergens do not need to be in bold on an edible label.
The second option is to use the word "Contains" followed by the name of the food source from which the major food allergen is derived, immediately after or adjacent to the list of ingredients, in a font size that is the same font size used for the list of ingredients.
For example, after the list of ingredients, the following statement would appear: Contains: Wheat, Milk, Pecans, Egg, and Soy
4. Source of Hemp. Ingredient lists must accurately list the source of or hemp used in the product. For example: “hemp extract,” “hemp concentrate,” or “hemp flower.”
Gluten-Free
Gluten is the protein that occurs naturally in wheat, rye, barley, and crossbreeds of these grains. Although certain grains may contain gluten, some grains can be made gluten-free. An ingredient that has been derived from a gluten-containing grain can be labeled as "gluten-free" if it has been processed to remove the gluten and use of that ingredient results in the presence of less than 20 parts per million (ppm) gluten in the food. The "gluten-free" claim is a voluntary one, however, licensees and registrants who decide to use this term are responsible for using the claim in a truthful and not misleading manner, and for complying with the requirements established by the U.S. Food and Drug Administration.
Gluten-free means that the food either is inherently gluten free or does not contain an ingredient that is: (1) a gluten-containing grain (e.g. Spelt wheat); (2) derived from a gluten-containing grain that has not been processed to remove gluten (e.g. Wheat flour); or (3) derived from a gluten-containing grain that has been processed to remove gluten (e.g. Wheat starch), if the use of that ingredient results in the presence of 20 parts per million (ppm) or more gluten in the food. Any presence of gluten in the food must be less than 20 ppm.